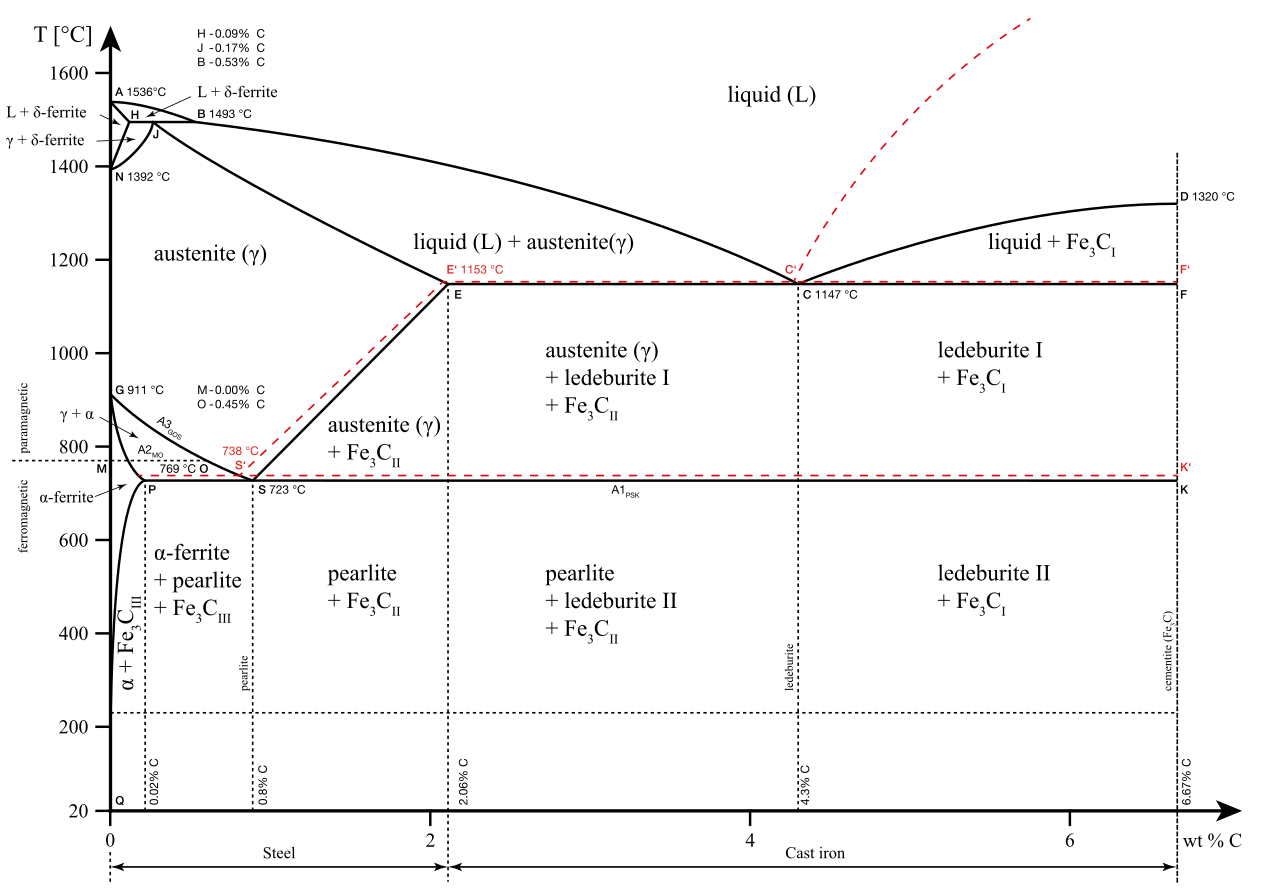
The term eutectic describes the property of a solid mixture to melt at a temperature that is lower than the melting point of its constituents. The word stems from ancient Greek εὔ-τηκein, “good-melting”. The steel-carbon alloy presents a eutectic point at 4.3% carbon, used for casting purposes.
The term was coined in 1883 by British physicist Frederick Guthrie: “I shall use it, and I should like it to be used by others, for bodies made up of two or more constituents, which constituents are in such proportion to one another as to give to the resultant compound body a minimum temperature of liquefaction - that is, a lower temperature of liquefaction than that given by any other proportion.” (Philosophical Magazine Series 5, Volume 17, 1884 - issue 108)